What are Zeolites?
Zeolites are microporous, aluminosilicate minerals commonly used as commercial adsorbents. Many occur naturally as minerals, and are extensively mined in many parts of the world. Others are synthetic, and are made commercially for specific uses, or produced by research scientists trying to understand more about their chemistry.
Because of their unique porous properties, Zeolites are widely used in industry for water purification, as catalysts, for the preparation of advanced materials and in nuclear reprocessing. They are used to extract nitrogen from air to increase oxygen content for both industrial and medical purposes. Their biggest use is in the production of laundry detergents. They are also used in medicine and in agriculture.
major uses are in petrochemical cracking, ion-exchange (water softening and purification), and in the separation and removal of gases and solvents. Other applications are in agriculture, animal husbandry and construction. They are often also referred to as molecular sieves.
Zeolites are microporous crystalline solids with well-defined structures. Generally they contain silicon, aluminum and oxygen in their framework and cations, water and/or other molecules within their pores. Many occur naturally as minerals, and are extensively mined in many parts of the world. Others are synthetic, and are made commercially for specific uses, or produced by research scientists trying to understand more about their chemistry.
Because of their unique porous properties, zeolites are used in a variety of applications with a global market of several milliion tonnes per annum. In the western world, major uses are in petrochemical cracking, ion-exchange (water softening and purification), and in the separation and removal of gases and solvents. Other applications are in agriculture, animal husbandry and construction. They are often also referred to as molecular sieves.
Framework Structure
A defining feature of zeolites is that their frameworks are made up of 4-connected networks of atoms. One way of thinking about this is in terms of tetrahedra, with a silicon atom in the middle and oxygen atoms at the corners. These tetrahedra can then link together by their corners (see illustration) to from a rich variety of beautiful structures. The framework structure may contain linked cages, cavities or channels, which are of the right size to allow small molecules to enter - i.e. the limiting pore sizes are roughly between 3 and 10 Å in diameter.
In all, over 130 different framework structures are now known. In addition to having silicon or aluminium as the tetrahedral atom, other compositions have also been synthesised, including the growing category of microporous aluminophosphates, known as ALPOs.
Catalysis
Zeolites have the ability to act as catalysts for chemical reactions which take place within the internal cavities. An important class of reactions is that catalysed by hydrogen-exchanged zeolites, whose framework-bound protons give rise to very high acidity. This is exploited in many organic reactions, including crude oil cracking, isomerisation and fuel synthesis. Zeolites can also serve as oxidation or reduction catalysts, often after metals have been introduced into the framework. Examples are the use of titanium ZSM-5 in the production of caprolactam, and copper zeolites in NOx decomposition.
Underpinning all these types of reaction is the unique microporous nature of zeolites, where the shape and size of a particular pore system exerts a steric influence on the reaction, controlling the access of reactants and products. Thus zeolites are often said to act as shape-selective catalysts. Increasingly, attention has focused on fine-tuning the properties of zeolite catalysts in order to carry out very specific syntheses of high-value chemicals e.g. pharmaceuticals and cosmetics.
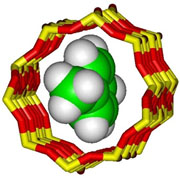 The shape of para-xylene means that it can diffuse freely in the channels of silicalite
Adsorption and Separation
The shape of para-xylene means that it can diffuse freely in the channels of silicalite
Adsorption and Separation
The shape-selective properties of zeolites are also the basis for their use in molecular adsorption. The ability preferentially to adsorb certain molecules, while excluding others, has opened up a wide range of molecular sieving applications. Sometimes it is simply a matter of the size and shape of pores controlling access into the zeolite. In other cases different types of molecule enter the zeolite, but some diffuse through the channels more quickly, leaving others stuck behind, as in the purification of para-xylene by silicalite.
Cation-containing zeolites are extensively used as desiccants due to their high affinity for water, and also find application in gas separation, where molecules are differentiated on the basis of their electrostatic interactions with the metal ions. Conversely, hydrophobic silica zeolites preferentially absorb organic solvents. Zeolites can thus separate molecules based on differences of size, shape and polarity.
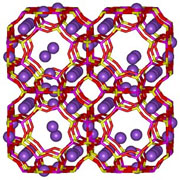 Sodium Zeolite A, used as a water softener in detergent powder
Ion Exchange
Sodium Zeolite A, used as a water softener in detergent powder
Ion Exchange
The loosely-bound nature of extra-framework metal ions (such as in zeolite NaA, right) means that they are often readily exchanged for other types of metal when in aqueous solution. This is exploited in a major way in water softening, where alkali metals such as sodium or potassium prefer to exchange out of the zeolite, being replaced by the "hard" calcium and magnesium ions from the water. Many commercial washing powders thus contain substantial amounts of zeolite. Commercial waste water containing heavy metals, and nuclear effluents containing radioactive isotopes can also be cleaned up using such zeolites.
Zeolites and the Environment
Zeolites contribute to a cleaner, safer environment in a great number of ways. In fact nearly every application of zeolites has been driven by environmental concerns, or plays a significant role in reducing toxic waste and energy consumption.
In powder detergents, zeolites replaced harmful phosphate builders, now banned in many parts of the world because of water pollution risks. Catalysts, by definition, make a chemical process more efficient, thus saving energy and indirectly reducing pollution. Moreover, processes can be carried out in fewer steps, miminising unecessary waste and by-products. As solid acids, zeolites reduce the need for corrosive liquid acids, and as redox catalysts and sorbents, they can remove atmospheric pollutants, such as engine exahust gases and ozone-depleting CFCs. Zeolites can also be used to separate harmful organics from water, and in removing heavy metal ions, including those produced by nuclear fission, from water.
Zeolites in the UK
Zeolite science and technology has traditionally been very strong in the UK. Partly this has been due to the scientific legacy of the late Professor Richard Barrer, the "father of zeolite science" who, during a career of over 50 years in various British universities, laid the foundations for the study of zeolites and discovered many of their important properties. The prominence of the UK has also been associated with the strength of its chemical industry, particularly in areas where zeolites have applications, such as petrochemicals, detergents, fine chemical synthesis and nuclear processing. Many British companies continue to have major R&D projects in these areas.
R.G. Bell, May 2001
*upfile/cp/16824213753.jpg*upfile/cp/16824213759.JPG*upfile/cp/1682421385.JPG*upfile/cp/16824213815.JPG*upfile/cp/16824213825.JPG*upfile/cp/16824213830.JPG

